Specification of health data transfer from devices to DiGA (§ 374a SGB V)
Seiteninhalt:
The range of medical aids and implants that could potentially be affected by § 374a SGB V is broad and diverse. It includes items such as blood pressure cuffs, blood glucose meters, real-time continuous glucose monitoring (rtCGM) devices, pacemakers, and neurostimulators.
In principle, different approaches are possible for specifying the FHIR interfaces used for data exchange between medical aids and DiGA. These approaches range from defining a modular, device-independent toolkit of FHIR building blocks to creating specific FHIR implementation guides for each kind of medical aid.
The modular “toolkit” approach was discarded. Even though this approach provides a high degree of flexibility using only few rather generic FHIR profiles, giving responsibility for the assembly and further constraining of these building blocks to manufacturers may lead to incompatible resource definitions even for rather similar data sets (e.g. there are at least three different ways for giving provenance to a device data item): compliant data on the producers’ side may not be interoperable for data consumers. On the other hand, device-specific implementation guides were also considered unsuitable. This is because they take the perspective of data producers as the standard, while the law clearly requires alignment with the intended purposes of DiGA as consumers of device data.
To meet this legal requirement – namely, to design the interface according to the processing purposes of the device data consumers – the approach outlined in the following diagram is used.
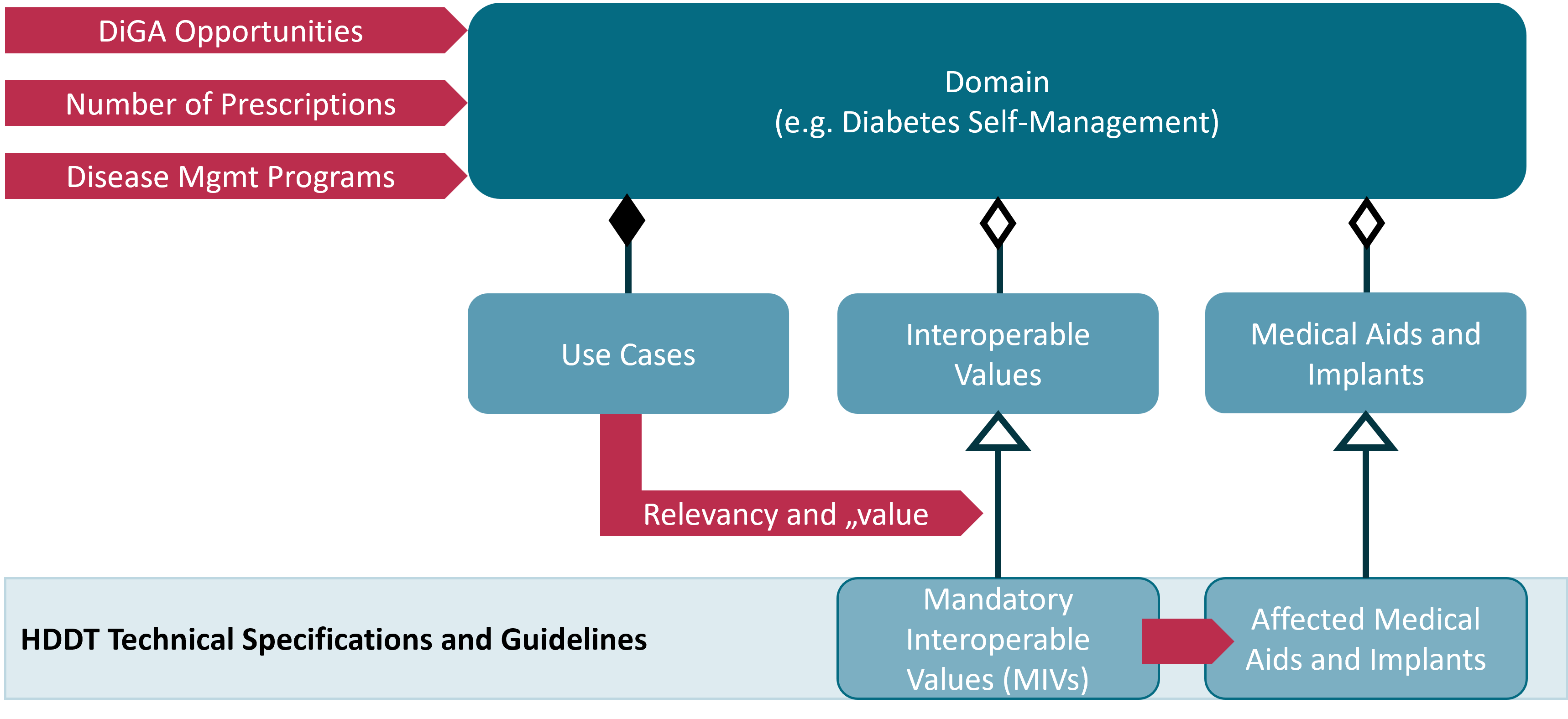
The selection of domains to be prioritized considered prescription numbers of certain medical aids as well as the potential of DiGA to support therapy management from a patient’s perspective. It was also important to initially choose domains that overlap with Disease Management Programs (DMP). Starting in 2027, the concept of “digital DMPs” will provide an innovation-friendly framework for the use of DiGA in the care of people with chronic diseases. Having access to device data will help to position innovative DiGA in this new regulatory environment.
For each domain, three aspects were analyzed: medical aids, interoperable values, and use cases. Each aspect was understood as a perspective from which the other two aspects could also be considered.
Based on these analyses, interoperable values in terms of their contribution to the use cases are evaluated. It is considered which properties of the values are important for the use cases and how the various concrete data can be assigned to these values. From this, a manageable set of particularly relevant interoperable values was conceptually defined for each domain. These are called “mandatory interoperable values” (MIVs). Every medical aid or implant that processes one or more MIVs is a potential HDDT certification relevant system that is obliged to implement the HDDT API for the provided MIVs.
MIVs are “conceptually defined” through their properties and purposes within the context of typical DiGA device data processing scenarios. This kind of rather logical than technical definition reflects the legal obligation from § 374a SGB V that a DiGA must only process device data if and only if this data is needed for the intended purposes of the DiGA.
Example: Blood Glucose Measurement and Continuous Glucose Measurement are two different MIVs: While the first is measured ad hoc and usable for clinical decisions the second is measured continuously in interstitial fluid (ISF) and suitable for calculating key indicators for the status of the treatment (e.g. %TIR and GMI). These different properties and contexts of use are also reflected in the considered use cases, where a DiGA can typically make use of only one or the other MIV.
Once a MIV is defined, a MIV-specific HDDT Observation Profile is specified. This profile constrains the FHIR Observation resource definition to match the requirements and specialties of the MIV. Measured values for MIVs are made available by the backend of a medical aid or implant through the standard FHIR RESTful read and search interactions.
Some MIVs are the basis for aggregated data. Such data is provided to DiGA as structured, standardized reports. Depending on the report’s content and structure, a dedicated FHIR profile (e.g. based on Observation or DiagnosticReport resource definitions) and a dedicated FHIR operation is defined for each report.
HDDT Observation Profiles and profiles for structured reports are managed by gematik and made available as FHIR StructureDefinition resources. FHIR operations for fetching a structured report are defined using formal definitions of input parameters and possible results.
Each MIV is expressed as a FHIR ValueSet which is managed with the ZTS (German Central Terminology Server). The LOINC codes within a MIV’s ValueSet are the only values allowed for the code element that classifies an FHIR Observation resource that holds a measured value for the MIV.
Example: The MIV “Continous Glucose Measurement” is expressed through the ValueSet “Continuous Glucose Measurement from LOINC”. This ValueSet includes a set of LOINC codes for typical measurements of this kind (e.g. 99504-3: Glucose [Mass/volume] in Interstitial fluid). The corresponding HDDT Observation Profile “HDDT Continuous Glucose Measurement.” constrains the code element of the FHIR Observation resource to only allow these LOINC codes. Continuously measured glucose values can be aggregated to key metrics such as the Glucose Management Index (GMI) and times in various ranges. For the MIV “Continous Glucose Measurement” these key metrics are summarized in a single report, the HDDT CGM Summary Report which builds upon an existing HL7 standard profile for rtCGM data.
The figure below summarizes the interplay of MIV-specific FHIR profiles (yellow), MIV definitions (green) and measured MIV resources (blue).
The table below lists the use cases, medical aids, and interoperable values that have been assessed so far. Interoperable values in fat letters are MIVs of the first specification version 1 (MVP). Medical aids in fat letters are devices that presumably are certification relevant systems with respect to the identified MIVs.
| Domain | Use Cases | Medical Aids | Interoperable Values |
|---|---|---|---|
| Diabetes Self-Management | • DiGA for supporting the adaptation of basal insulin rate • DiGA with digital diabetes diary • DiGA for simple asynchronous telemonitoring • DiGA for the prevention of glucose peeks |
• blood glucose meter • rtCGM • smart pen • insulin pump • … |
• Blood Glucose Measurement • Continuous Glucose Measurement • insulin intake |
| Respiratory Monitoring | • DiGA to support treatment of asthma bronciale • DiGA for compliance monitoring • DiGA to support treatment of sleep apnea |
• peak flow meter • spirometer • CPAP • APAP • … |
• PEF • FEV1 • FVC • FEV6, … |
| Simple Cardiac Monitoring | to be defined | • blood pressure cuff • scale • … |
• Blood Pressure • Pulse • body weight |
Remark: For the current ballot version of the HDDT specification, only the Diabetes Self-Management domain is subject of the balloting.