Specification of health data transfer from devices to DiGA (§ 374a SGB V)
Seiteninhalt:
§ 374a SGB V requests vendors of medical aids and implants to provide medical device data for authorized digital health applications (DiGA) through a standardized backend API. This API is specified by the Health Device Data Transfer (HDDT), which provides normative definitions for the data transfer itself as well as for accomplishing security services.
This chapter provides a logical view on the HDDT ecosystem and by this gives an overview of the specification-relevant aspects. It introduces the core building blocks and their interactions. It also defines the data that is subject to the HDDT specification and the security services that are required for securely grant access to a patient’s device data.
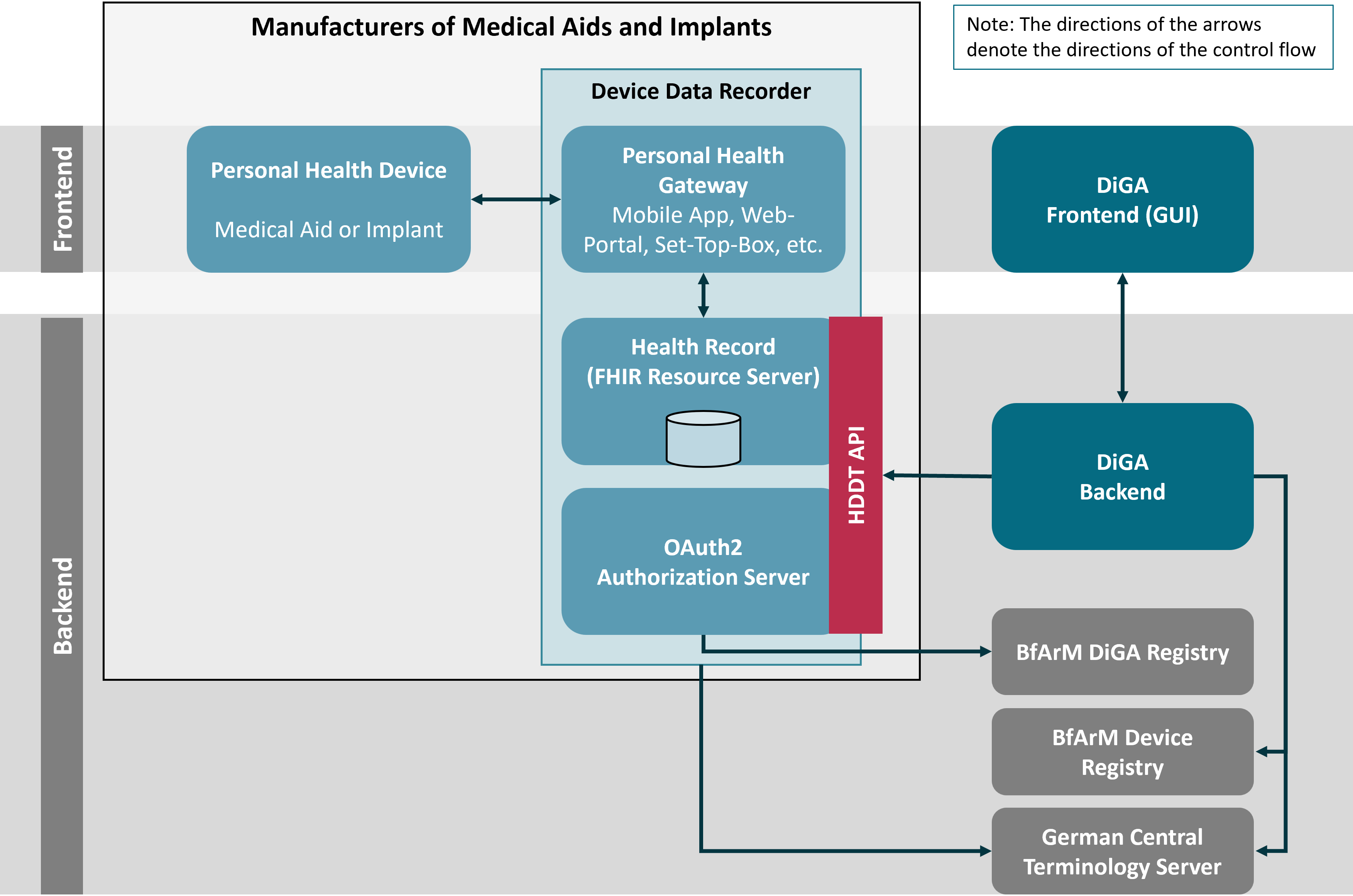
For the HDDT specification, the HDDT ecosystem is divided into several logical building blocks. For the components operated by the manufacturers of the medical aids’ and implants’ devices, frontends and backend systems, the HDDT specification follows the logical decomposition defined by the Continua Health Alliance and adopted by the HL7 Personal Health Device WG:
The legislative rational for § 374a SGB V explicitly defines the HDDT API for disclosing data from medical aids and implants to eligible DiGA as a backend API. Therefore DiGA MUST access device data only through the Health Record. For HDDT the Health Record acts as a FHIR Resource Server that implements standard FHIR RESTful interactions that provide access to device data as FHIR resources. In this specification the term Health Record is used synonymously with FHIR Resource Server, with the first preferably used when the perspective of the data controlling entity is taken and the second being preferred when the perspective of the data provider to DiGA is taken.
Access to the FHIR Resource Server is restricted to authorized DiGA which are listed with the BfArM DiGA-VZ (BfArM DiGA Registry). DiGA can proof authorization to the FHIR Resource Server through an OAuth2 Access Token. This token is issued by an OAuth2 Authorization Server which is operated by the data controller, which is the owner of the Health Record. The Authorization Server not only considers a DiGA’s permissions as recorded in the BfArM DiGA-VZ but also takes care that the patient has given valid consent for sharing his health data with authorized DiGA (see Pairing for details).
In this specification the combination of Personal Health Gateway, Health Record (Resource Server), and OAuth2 Authorization Server is called the Device Data Recorder (see section on Certification Relevant Systems for a discussion on various vendor constellations). The manufacturer of the Device Data Recorder is responsible for implementing the HDDT API which consists of the FHIR based API of the FHIR Resource Server and the OAuth2 compliant API of the OAuth2 Authorization Server.
In order to securely operate the HDDT API, Manufacturers of Device Data Recorders MUST
DiGA-VZ and HIIS-VZ are not part of the HDDT specification and will be defined in dedicated technical specifications by BfArM. The German Central Terminology Service ZTS is defined and operated independently from HDDT. A non-normative description of these three services and their interfaces is given in section BfArM Registries and ZTS.
The figure below shows how these logical building blocks interact with each other.
Subject of the data transmission as requested by § 374a SGB V are measured data, aggregated data and configuration data:
The figure below shows the relationship between Personal Health Devices, measured data and aggregated data for the domain “Diabetes Self-Management”. Relationships between Personal Health Devices and measured data is n:m which means that a Personal Health Device could provide many kinds of measured data while each measured data may be provided by multiple kinds of Personal Health Devices.
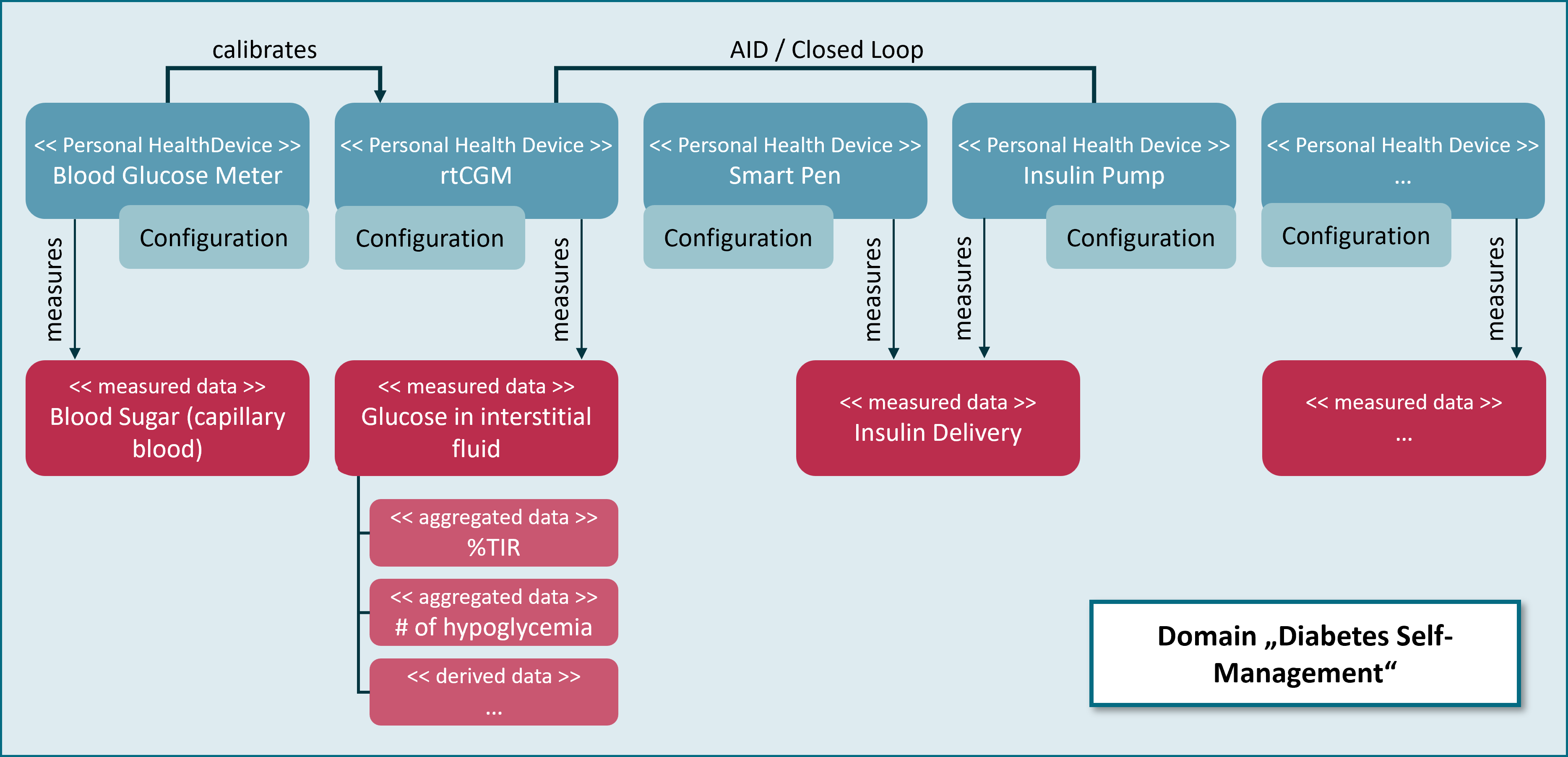
Further information on devices and data is given in the sections Information Model and Retrieving Data. The technical specifications of the FHIR resources that measured data are domain specific. FHIR implementation guides for various kinds of data related to glucose measurements can be found in the chapter MIV-Specific APIs.
As stated, the relationship between Personal Health Devices and measured data is many-to-many. The same holds for DiGA and required data; e.g. a diabetes DiGA may use blood glucose values from glucometers as well as data about insulin injections from SmartPens and body weight measurements from a digital scale for it’s legitimate purposes. For matching the data that is requested by a DiGA with the data that is provided by Personal Health Devices via Device Data Recorders, the HDDT specification introduces the notion of Mandatory Interoperable Values (MIVs). A MIV is a conceptual definition of a certain kind of device data that is processed by DiGA. A “conceptual definition” defines data by its properties and purposes within the context of a DiGA device data processing scenario. An example for a MIV is Blood Glucose Measurement. This MIV is measured from blood according to a care plan (e.g. taking measurements before main meals) or on demand (e.g. a patient detecting symptoms of hypoglycemia). Values are usable for clinical decisions. Continuous Glucose Measurement is another example for a MIV. This one is measured continuously in interstitial fluid (and in the future probably even through optical means) and suitable for calculating key indicators for the status of the treatment (e.g. times in ranges and glucose management indicator) but values are not as acurate as Blood Glucose Measurements. Even though both of these two MIVs express glucose values, they both will presumably be used in different use cases by different DiGA.
The DiGA Verzeichnis and HIIS-VZ at BfArM record for each DiGA, each Personal Health Device and each Device Data Recorder the MIVs which are processed. This allows for discovering which device data providers match the demands of a certain DiGA and which DiGA can process the data provided by a certain Personal Health Device via a certain Device Data Recorder.
It is assumed that the DiGA and Device Data Recorder do not have a common shared patient identifier. Even though the German GesundheitsID (health ID) is a global patient ID which could be used for identifying the patient, recently neither DiGA nor Personal Health Devices or Device Data Recorders are allowed to obtain this identifier from the patient’s health insurance. Therefore, if a patient allows a DiGA to request device data from a Personal Health Device through a Device Data Recorder, DiGA and Device Data Recorder must first link their respective patient accounts. This is done by the DiGA calling an API at the Device Data Recorder’s OAuth2 Authorization Server. At this point the patient is already logged in with the DiGA while the OAuth2 Authorization Server forces the patient to as well log in with the Device Data Recorder’s Personal Health Gateway that controls the Personal Health Device. The patient is now simultaneously activated with both actors, which allows DiGA and Device Data Recorder to share a common identifier that unambiguously identifies the combination of patient, DiGA and Device Data Recorder (“pairing-ID”).
The pairing flow is based on the OAuth2 standard. The MIV that defines the measured data which is requested by the DiGA is encoded as an OAuth Scope. Upon pairing the Device Data Recorder validates the requested scope with the DiGA-VZ at BfArM. This registry lists for all DiGA the MIVS - represented by a value set of LOINC codes - that the DiGA is allowed to process. The committed OAuth2 Scope is then encapsuled with an access token that is handed back to the DiGA by the OAuth2 Authorization Server. Whith every request for data the DiGA provides the Device Data Recorder with this access token as a proof of authorization. Access token can be renewed using the standard means of OAuth2.
The sequence diagramm below sketches the pairing flow between DiGA and Device Data Recorder followed by a request for device data using a FHIR RESTful interaction.
Further information on the OAuth2 Authorization Server and the authorization flow is given in the section Pairing. The technical specification of the HDDT profile on the OAuth2 standard can be found in the section Authorization Server. The required validation of access tokens for accessing FHIR resources from the Device Data Recorders FHIR Resource Server is specified in the section Generic FHIR Resource Server API.